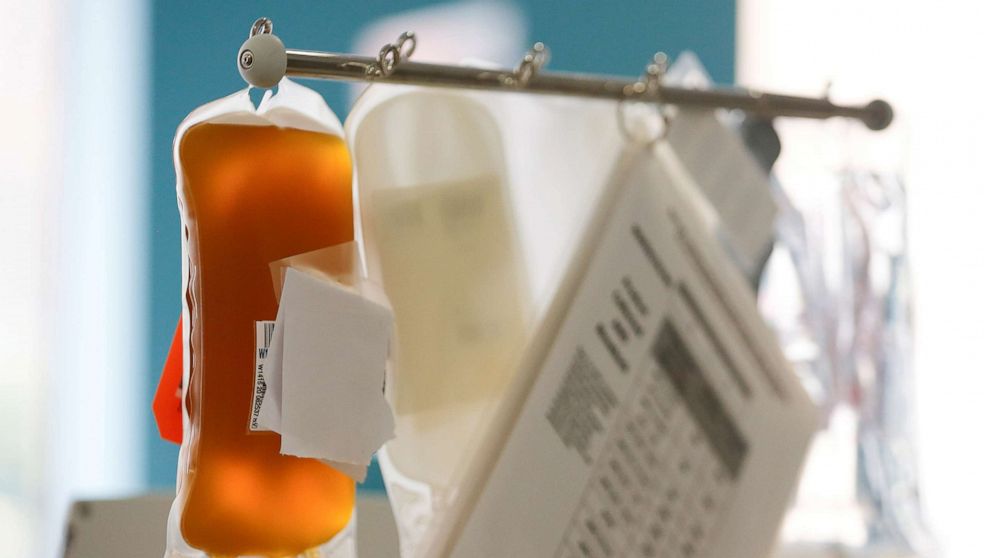
The FDA is issuing an emergency authorization for convalescent plasma treatment in hospitalized COVID-19 patients, agency officials told reporters on a call Sunday, ahead of President Donald Trump's late afternoon press briefing.
"We're encouraged by the early promising data that we've seen about convalescent plasma," FDA Commissioner Dr. Stephen Hahn said, adding that data shows it "has the potential to help treat those who are suffering from the effects of getting this virus," but that "this is not the end" of their research and work -- and that they will update their Emergency Use Authorization (EUA) as appropriate.

No comments:
Post a Comment